


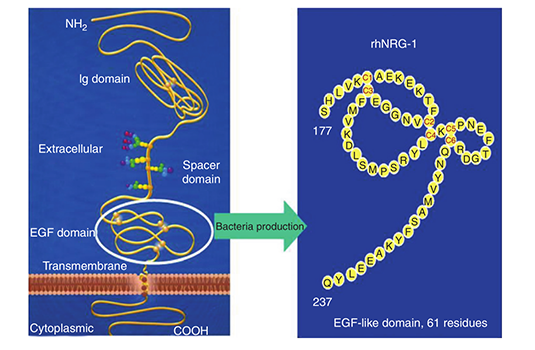
Zensun began its lab on Bibo Road, Zhangjiang High-tech Park. Here Zensun finished preclinical research for the earliest “First-in-Class” product NRG-1 and rhErbB3-f in China.
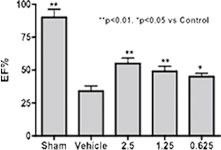
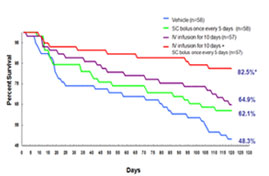
HF rat model induced by LAD-ligation. Left: 24 hours after 7 days IV infusion of Neucardin, cardiac function of all dosing groups was found to be improved. Right: survival rate was observed to be improved after either IV or SC infusion at an optimal dose for different periods of time.

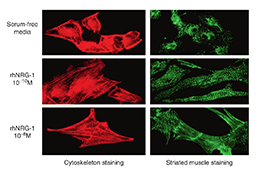
In April 2003, Zensun completed the rh-Neuregulin-1 preclinical study. . The was a rigorous and scientific preclinical study of multiple pathological animal models of heart failure caused by ischemic, dilated, rapid pacing and viral infections. rh-Neuregulin-1 initially demonstrated that it repairs damaged myocardial cell structures caused by ischemia, hypoxia, and viral infections, enhances cardiac function, improves cardiac hemodynamics, but also prolongs the lives of animals with heart failure. This finding was later published in “The American Journal of Cardiology”, the most authoritative independent medical journal in regards to the cardiovascular field.


Zensun USA offices are located at 12760 High Bluff Dr., Ste360. Many world famous biotech companies gather in San Diego.
Zensun’s headquarters and research center are located in Zhangjiang High-tech Park, over thirteen thousand square meters to perform cytology, molecular biology and pharmacology.

From 2006 to 2007, Zensun purchased Shanghai Dongxin Biotechnology Co., Ltd. for it’s factory, equipment and land in the central area of Zhangjiang. It is now Zensun’s headquarter and research center.

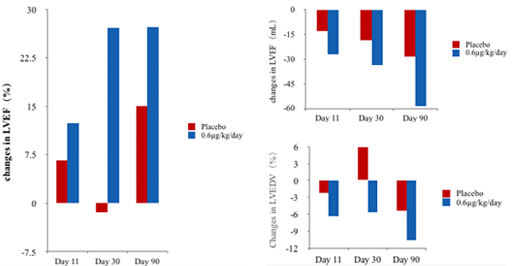
Phase II clinical trial has demonstrated that Neucardin can increase the LVEF of HF patients significantly (left), and also improves cardiac function and continuously reverses ventricular remodeling.

In April 2009, Zensun China completed Neucardin® phase II clinical trial ZS-01-207, which showed that patients’ NT-proBNP was significantly reduced by Neucardin®, which improved long-term prognosis. The following clinical trial, ZS-01-304, produced data supporting this finding.
In July 2009, based on data generated from Chinese and Australia clinical trials, FDA permitted Zensun USA to start phase II clinical trials in the U.S..


Immunogenicity methodology of NRG-1 and animal prausnitz-kstner reactions were done at a former lab of Zensun USA located at 6408 Cornerstone ct, Ste B.
 Zensun’s headquarter in Shanghai.
Zensun’s headquarter in Shanghai.



Zensun’s research center in Shanghai, Zhangjiang.

Zensun obtained FDA permission to perform phase III clinical trial of NRG-1.



Zensun announces its initial public offering at NEEQ(National Equities Exchange and Quotations) hall . Dr. Mingdong Zhou, President of Zensun, addressed the audience.
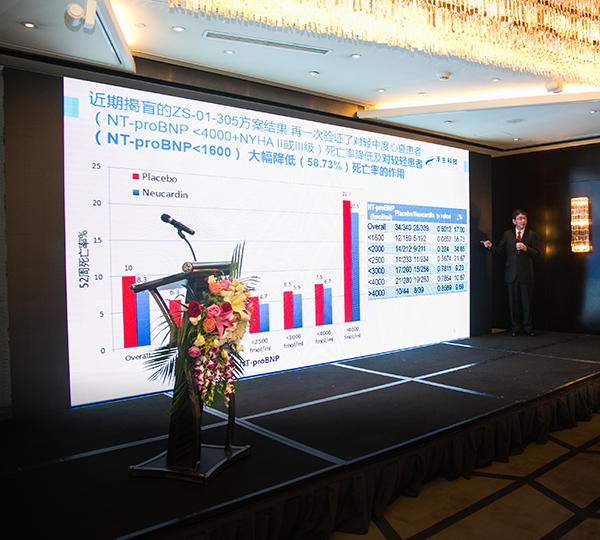
ZS-01-305 interim analysis unblinded conference. Dr. Mingdong Zhou, President of Zensun, showed inspiring result of ZS-01-305.



ZS-01-306 launch meeting. Professor Runlin Gao (PI of Neucardin®s Phase II& III Clinical Trial) presents the history of Neucardin®s clinical trial.



地址:上海市浦东张江高科技园区居里路68号沪ICP备06030993号-3