Neucardin®(HFrEF)
Recombinant human neuregulin-1 (Neucardin®), developed by Zensun, is a novel genetically engineered biologic used in the treatment of mild and moderate chronic heart failure (CHF). The advantage of Neucardin® is that it can specifically target the cardiac muscle cell, repair the cell structure and improve function during heart contraction and relaxation, thereby improving cardiac function and reversing pathological ventricular remodeling,and significantly reducing death rate and readmission rate while improving the quality of life.
Neucardin® is a recombinant protein biologic, derived from the bioactive peptide released from human neuregulin-1. It binds specifically to the ErbB4 receptor on the cardiomyocyte membrane, mediating the formation of ErbB2/ErbB4 heterodimers and activating the downstream MAPKs and PI3K signaling pathways. The activated pathways are critical for the survival, differentiation, structural reorganization and contraction of cardiomyocytes. For example, research on the cellular level revealed that Neucardin® enhances the intercalated disk connections between cardiomyocytes and thus reorganized the cell structure of cardiomyocytes. Animal efficacy tests also showed that Neucardin® improves heart function and hemodynamics, mainly by decreasing the damage caused by ischemia, hypoxia and virus infection, and repairing the cell structure of impaired cardiomyocytes.
Zensun has now shown for the first time that Neucardin® improves heart function by activating two critical signaling pathways, one involving the increased expression of cardiac specific myosin light chain kinase (cMLCK) and the other involving the decreased expression of myocardial protein phosphatases (PP1 and PP2). Increased expression of cMLCK stimulates phosphorylation of myosin light chain (MLC2v), followed by the promotion of sarcomere reorganization and the improvement of contractive function of cardiac muscle. Decreased expression of PP1 and PP2 suppresses phosphorylation of cardiac phospholamban (PLB), activating the sarcoplasmic reticulum Ca2+-ATP enzyme (SERCA2a) that regulates the Ca2+ cycle, and thereby improving the contraction and relaxation function of the heart.
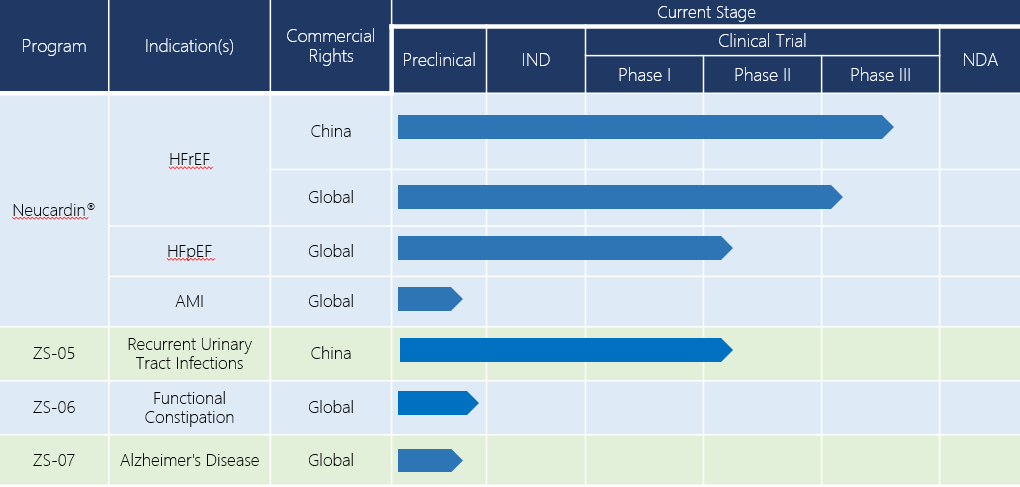
Over the past 20 years, Zensun has conducted and accomplished phase I and phase II clinical trials of Neucardin® in China, Australia and the U.S. These trials evaluated the efficacy and safety of Neucardin® in both healthy subjects and patients with CHF. The unambiguous conclusion of the phase II clinical trials conducted is that Neucardin® has a prominent therapeutic effect for patients with mild to moderate CHF (NYHA II – III). In addition to the existing standard treatment, administration of Neucardin® can significantly improve the left ventricular ejection fraction (LVEF, with the absolute value improved 3-5 %), reduce systolic volume, repair myocardial cells and reverse pathological ventricular remodeling. Clinical trials conducted in China and the U.S. further revealed that Neucardin® can also improve the athletic performance and living quality of patients, while reducing death and readmission rates. At present, Neucardin® is in its phase III clinical trial in China, and a global phase III clinical trial is also planned for launch.
Comprehensive and strict intellectual property protection of Neucardin® has been established by Zensun in the major countries and regions of the world, covering, but not limited to, its mechanism, indications, administration methods, dosage, pharmaceutical preparations, and downstream targets.
In conclusion, Neucardin® is a promising innovative biologic for CHF treatment because of its novel mechanism, prominent efficacy, excellent safety profile, and comprehensive patent protection. The innovative and unique mechanism of action of Neucardin® also makes it a biologic of ground-breaking significance covering the current severe shortage of options in this field, and provides more effective treatment for a devastating disease in combination with existing CHF drugs.
Expanded Access Program Policy
In USA, Zensun USA is developing Neucardin® for the treatment of patients with Chronic Heart Failure. Our focus as a company is to bring new medicines to patients using sound scientific and medical processes. We encourage patients seeking new treatments for serious diseases to discuss with their treating physician the possibility of participating in a clinical trial.
Phase 3 clinical trials are designed to establish the safety and effectiveness of experimental therapies to allow review by Regulatory Agencies such as the US Food and Drug Administration (FDA) to approve drugs for marketing. There is a period of time between when Phase 3 studies have been completed and a drug is approved that the company believes is an appropriate time for distribution of drugs through Expanded Access (also referred to as compassionate use). At the current stage of development, we believe it is premature to offer this investigational drug product for Expanded Access, and we are currently not able to respond to requests for our drug outside of clinical trials.
We expect this policy to maximize the resources we have available to accelerate the timelines for establishing the benefit-risk profile of the drug sufficient to determine when to update this policy and consider filing for drug approval with the FDA. We may update this policy in the future and appreciate your interest in Zensun USA.
Zensun USA, Inc. Expanded Access Policy (Version 1), August 21, 2019