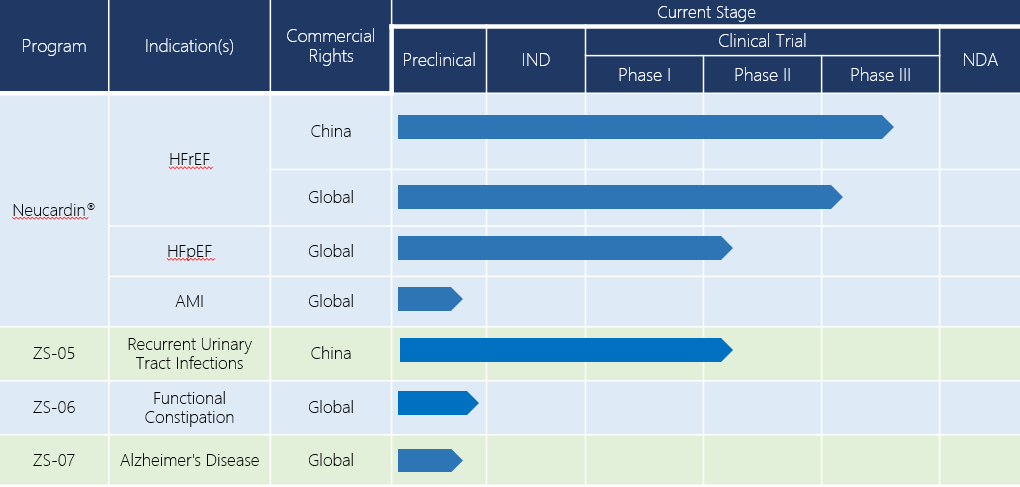
Neucardin®(HFpEF)
Heart failure with a preserved ejection fraction(HFpEF)is characterized by incomplete relaxation and reduced compliance of cardiomyocytes. HFpEF patients make up 50% of all chronic heart failure patients. There is no evidence that the drugs currently available could reduce the morbidity and mortality rates in HFpEF patients, until now.
The effect of Neucardin® on cardiomyocytes brings hope that there could be a treatment for HFpEF. Preclinical research shows that Neucardin® improves left ventricular compliance in spontaneously hypertensive rats, reduces Ca2+ overload of animal model, and results in a higher maximum speed of instant decrease of Ca2+ during diastole in rats with chronic heart failure induced by spontaneous hypertension. In Neucardin® clinical trials for HFrEF treatment, data shows that Neucardin® could reverse ventricular remodeling. Therefore, Neucardin® is very promising to be the first drug curing HFpEF in history. Now Zensun is preparing for a phase II clinical trial for this indication.
Expanded Access Program Policy
In USA, Zensun USA is developing Neucardin® for the treatment of patients with Chronic Heart Failure. Our focus as a company is to bring new medicines to patients using sound scientific and medical processes. We encourage patients seeking new treatments for serious diseases to discuss with their treating physician the possibility of participating in a clinical trial.
Phase 3 clinical trials are designed to establish the safety and effectiveness of experimental therapies to allow review by Regulatory Agencies such as the US Food and Drug Administration (FDA) to approve drugs for marketing. There is a period of time between when Phase 3 studies have been completed and a drug is approved that the company believes is an appropriate time for distribution of drugs through Expanded Access (also referred to as compassionate use). At the current stage of development, we believe it is premature to offer this investigational drug product for Expanded Access, and we are currently not able to respond to requests for our drug outside of clinical trials.
We expect this policy to maximize the resources we have available to accelerate the timelines for establishing the benefit-risk profile of the drug sufficient to determine when to update this policy and consider filing for drug approval with the FDA. We may update this policy in the future and appreciate your interest in Zensun USA.
Zensun USA, Inc. Expanded Access Policy (Version 1), August 21, 2019